The PureAire Combustible Gas Detector is a safety device designed to detect flammable gases in an area. This early warning system is vital in identifying potential dangers that could go unrecognized until it is too late. Excessive levels of combustible gases, whether due to a gas leak or an accumulation of gases during manufacturing processes, can be overlooked […]
READ MORE
Carbon Dioxide Monitors A CO2 monitor, also known as a carbon dioxide monitor, is essential equipment to help maintain healthy indoor air quality. It detects and measures the concentration of CO2 in the air. PureAire stands at the forefront of this field, offering high-quality carbon dioxide monitoring and detection solutions for various applications. By alerting […]
READ MORE
Introduction Oxygen (O2) deficiency monitors, also known as oxygen depletion monitors, are vital safety equipment used across a wide array of industries that help ensure personnel safety. These safety monitoring systems detect low levels of oxygen in the air and will trigger visual and audible alarms when oxygen concentrations drop to an unsafe level. Unsafe, […]
READ MORE
The evolution of toxic gas detectors is rooted in industrial safety, with milestones marking significant leaps in detection technology. In the early 20th century, electrochemical sensors were among the first devices designed to identify lethal gases in coal mines. The sensors relied on a chemical reaction between the toxic gas and an electrode, generating an electrical […]
READ MORE
Introduction Oxygen Depletion, also known as oxygen deficiency, is a serious safety issue that occurs when the oxygen concentration in an environment falls below the level necessary to sustain human life. The Occupational Safety and Health Administration (OSHA) defines an environment where oxygen levels fall below 19.5% as an oxygen-deficient atmosphere, which should be treated […]
READ MORE
Understanding Oxygen Deficiency The air we breathe is made up of 78% nitrogen, 21% oxygen, and trace amounts of other gases such as carbon dioxide, neon, and hydrogen. The oxygen level in the air we breathe is approximately 20.9%. A drop in oxygen levels below this percentage indicates an oxygen-depleted environment that poses significant health and […]
READ MORE
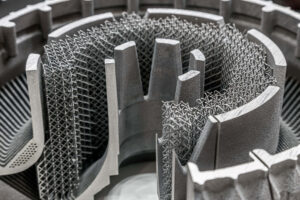
Metal 3D printing, also known as additive manufacturing, provides for the creation of complex metal parts by layering metal powders and, depending on the application, selectively sintering, fusing, or melting the powders using a high-powered laser or electron beam. This process offers numerous advantages over traditional manufacturing methods, including reduced waste, increased design freedom to […]
READ MORE
PureAire Monitoring Systems is excited to announce the launch of our latest offering—the PureAire CloudConnect module, which provides for internet connectivity (with Cloud storage capabilities) for our full line of Oxygen and Carbon Dioxide Monitors, as well as our Toxic and LEL Combustible Gas Detectors. Our new CloudConnect module will send continuous gas concentration data […]
READ MORE
What is a Biorepository? A biorepository, or “biobank”, is a specialized facility designed to store, archive, and distribute biological samples for research or clinical purposes. Biorepositories house biological samples, such as blood, plasma, urine, saliva, tissues, DNA, and organs, among other specimen types, collected from consenting individuals. Critical associated information, including relevant health information about […]
READ MORE